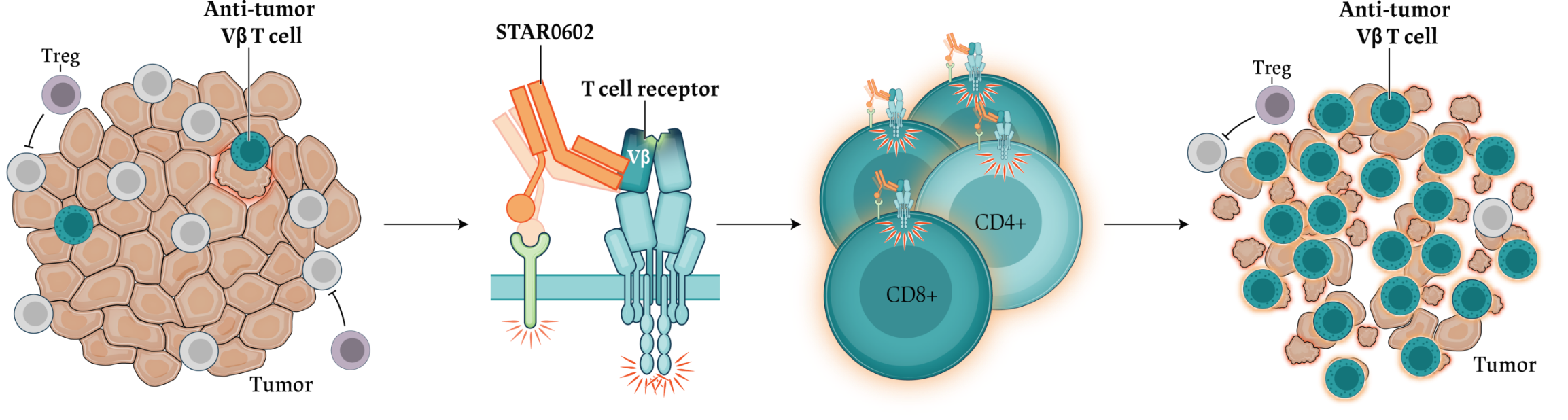
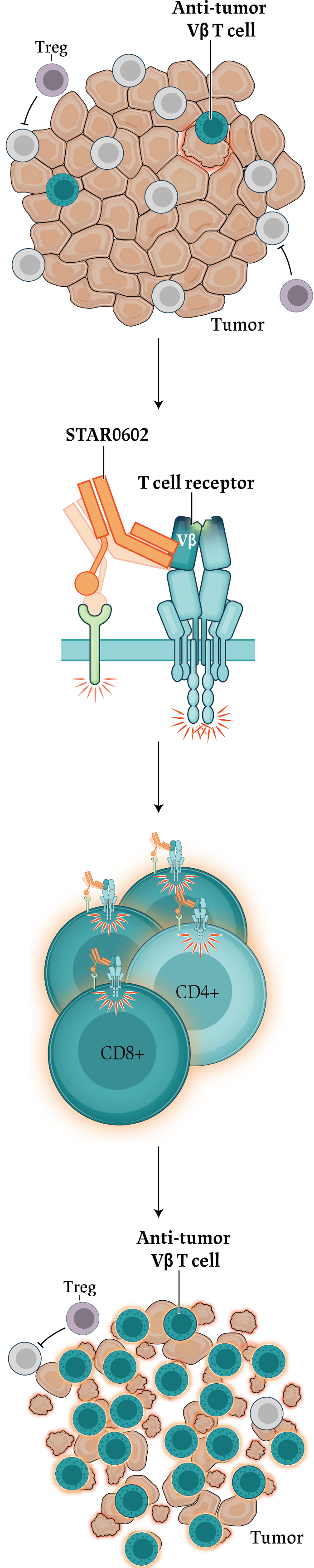
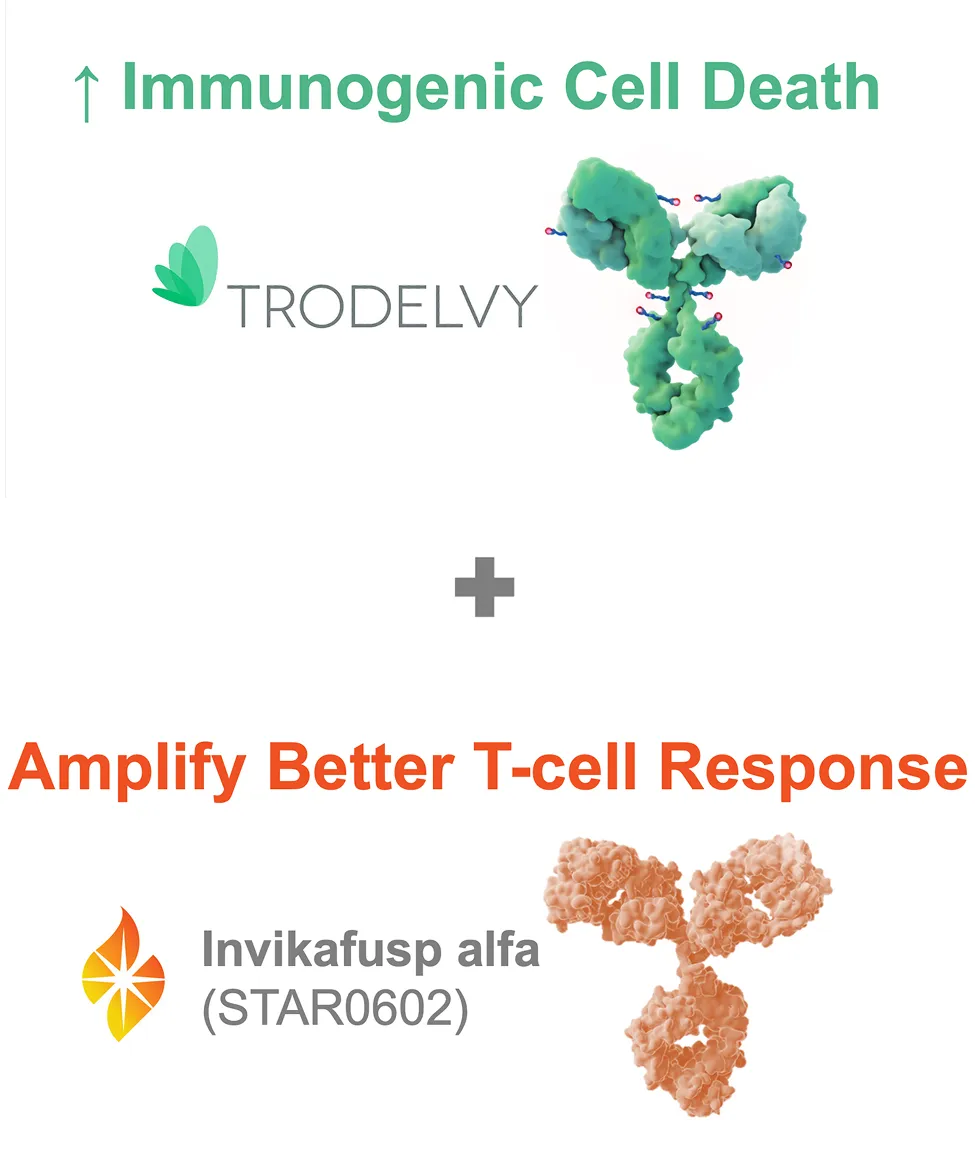
Powered for progress
and breakthrough.
Oncology
Oncology
Autoimmune
Oncology
Autoimmune
T cell
agonist)
Oncology
Wholly
owned
Oncology
Collaboration*
with
Oncology
Wholly
owned
Oncology
Partnered with

Oncology
Partnered with

T cell
engager)
Oncology
Wholly
owned
Oncology
Wholly
owned
Autoimmune
Wholly
owned
Oncology
Partnered with

Oncology
Partnered with

T cell
depleter)
Autoimmune
Wholly
owned
Autoimmune
(Discovery)
Wholly
owned
*Marengo is the sponsor of the clinical study and Gilead will provide the drug supply of TRODELVY®.